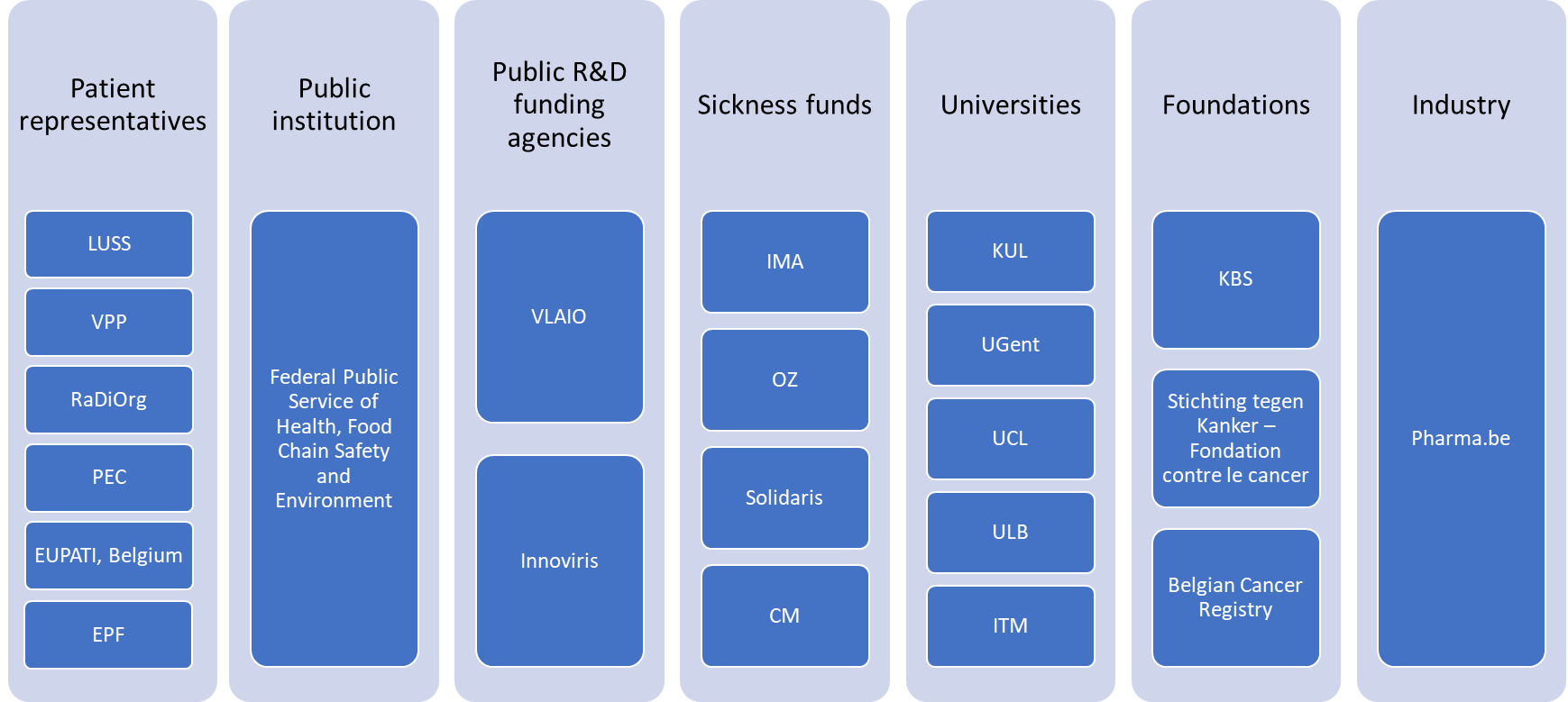
The national NEED advisory committee is composed of representatives of Belgian stakeholders, based on their expressed interest in the NEED project at the proposal stage:
- Patient representatives : LUSS, VPP, RaDiOrg, PEC, EUPATI.BE, European Patients' Forum (EPF)
- Public institution: Federal public service of Health, Food Chain Safety and Environment
- Public R&D funding agencies: Vlaams Agentschap Innoveren en Ondernemen (VLAIO), Innoviris
- Sickness funds: MLOZ, Christian Mutuality (CM), Solidaris, Intermutualistic Agency (IMA)
- Belgian Universities: Katholieke Universiteit Leuven (KULeuven), Ghent University (UGent), Université catholique de Louvain (UCL), Université Libre de Bruxelles (ULB), Institute of Tropical Medicine (ITM)
- Foundations: King Baudouin Foundation (KBS/FRB), Stichting tegen Kanker/Fondation contre le Cancer, Belgian cancer registry
- Pharmaceutical industry: Pharma.be
The international NEED advisory committee is composed of representatives of European (networks of) public agencies and institutions:
- International Networks : International Horizon Scanning Initiative (IHSI), European Social Insurance Platform (ESIP)
- Public institutions: Department of Health Ireland, Ministry of Health, Welfare and Sport (the Netherlands), Zorginstituut Nederland (the Netherlands), Haute Autorité de Santé (France), Austrian Federal Ministry of Social Affairs, Health, Care and Consumer protection, Austrian Social Insurance, Austrian Agency for Health Technology Assessment (AIHTA), National Public Health Institute Austria (GOEG), Directorate of Health Luxembourg, Finnish Medicines Agency (FIMEA), INFARMED (Portugal), National Institute of Pharmacy and Nutrition (OGYéI, Hungary), IQWIG (Germany), Ministry of Health Spain, Danish Medicine Agency (DKMA), Norwegian Institute of Public Health (NIPH) and the Norwegian Medicines Agency
- European Commission: DG Health and Food Safety and DG Research and Innovation
Meeting documents:

